Advancing the development of vaccines through recombinant technology


Amy Sheng (left) is Head of the CRO Program at Sino Biological US Inc. (PA, USA), and has expertise in cell, molecular biology, immunology and biochemistry. In this interview, Amy discusses the current landscape of vaccine development, how recombinant vaccines are developed, including the use of AI, and the future of the field.
What is the current status of vaccine development?
Vaccines come with an inherent societal benefit that goes beyond safeguarding individuals from serious infectious diseases. They also extend their protective shield to the entire community, thereby reducing the burden of hospitalizations, fatalities and healthcare expenses in the USA. As new and innovative vaccines continue to be developed, they hold the potential to provide even greater benefits to society.
The pursuit of vaccine development is a daunting and costly task, fraught with high risks. It starts with evaluating the available information on the disease burden and technical feasibility to gauge the initial product interest. If the decision to develop a vaccine is made, it typically requires a minimum of 10 years of investment to move the potential candidates from basic and applied research to licensure, production and eventual integration into the immunization delivery system.
Traditional injectable vaccines, administered into the muscle or under the skin, remain the most widely used method of vaccine delivery. This system dates back over a century, but newly designed needles and injection devices have made it easier and less painful for patients to receive injectable vaccines. Oral vaccines have become more popular in recent years. These vaccines are administered through the mouth and typically protect against diseases that affect the gastrointestinal tract, such as cholera, rotavirus and typhoid fever. Overall, oral vaccines are an important tool in the fight against infectious diseases, especially in areas where access to medical care and vaccination programs may be limited.
DNA and mRNA vaccine delivery systems have stepped into the spotlight since the COVID-19 pandemic, utilizing viral or bacterial genetic material to instigate an immune response from the body. In both systems, the body’s cells utilize genetic information presented in the vaccine to produce a viral protein ‘in-house’, which is then recognized by the immune system, triggering a stronger and more efficient response. DNA and mRNA vaccines allow for precise delivery of genetic information to the cells that need to produce the viral protein without the risk of infection from the actual virus.
According to the Pharmaceutical Research and Manufacturers Association of America (PhRMA) website, over 250 vaccine candidates are presently being developed, the majority of which target infectious diseases and cancers [1]. The vaccine industry in the USA is a well-established and successful sector that has introduced several new, advanced products to the market. This accomplishment owes much to the support of federal agencies, such as the US FDA, CDC, NIH, National Vaccine Program Office, etc., which play a central role in supporting critical components (research, development and production, public health education) of the vaccine enterprise.
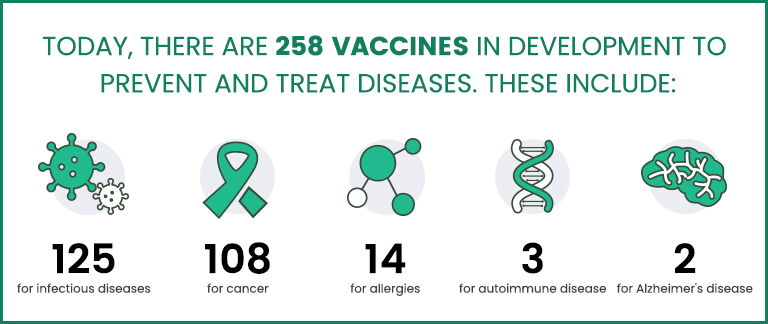
Fig. 1 New Era of Medicine: Vaccines [1]
How are recombinant vaccines developed?
Recombinant vaccines include several categories: recombinant protein vaccines, DNA vaccines and live recombinant vaccines using bacterial or viral vectors. The majority of vaccines currently being studied rely on the use of highly purified recombinant proteins or subunits of pathogens [2]. In this strategy, antigens are produced recombinantly in bacteria, yeast, mammalian cells or insect cells and formulated with other components such as adjuvants to create vaccines. One of the more famous examples of recombinant vaccines currently in use for humans is the vaccine against hepatitis B (HBV), created by expressing the hepatitis B surface antigen (HBsAg) in yeast cells. These antigens assemble into virus-like particles (VLPs) that are highly immunogenic, making the HBV vaccine incredibly effective. On the other hand, the use of mammalian systems has the advantage of enabling the secretion of the antigen into the culture supernatant, thus simplifying its purification process. Additionally, eukaryotic cellular machinery can perform post-translational modification of proteins, such as glycosylation [3]. Glycosylation leads to conformational changes in the protein, which can effectively increase the immune response. In the context of recombinant vaccine development, post-translational modifications offer advantages such as enhanced immunogenicity, increased stability, improved solubility and a more controlled activity of the antigenic proteins.
DNA vaccines involve the administration of a naked DNA plasmid directly into the muscle, which has the capability to provoke an immune response and provide protection against pathogens after challenge. Numerous pathogens, including influenza, HIV, malaria, tuberculosis and leishmaniasis have been targeted through antigen expression using DNA vaccines, successfully inducing immune responses against these causative agents in various animal models and even leading to protection in some instances. Nevertheless, although DNA vaccines have been demonstrated to be safe and well-tolerated, they have been found to be less immunogenic in non-human primates and humans.
What are the challenges in vaccine development?
Current vaccine strategies struggle to elicit significant cell-mediated immunity against intracellular pathogens that typically cause chronic infections. Live attenuated pathogen vaccines capable of provoking this type of response may present risks, such as virulence in susceptible hosts and potential reversal of attenuation, which cannot be ignored.
Recombinant vaccines, on the other hand, rely on one or multiple defined antigens to prompt immunity against the pathogen, either by administering them in the presence of adjuvants or by expressing them through harmless viral/bacterial vectors or plasmids. This technology enables the avoidance of potential concerns associated with vaccines based on purified macromolecules, such as the risk of co-purification of undesired contaminants or toxoid reversal to their toxigenic forms, as seen in diphtheria or tetanus toxoid vaccines. Additionally, recombinant vaccine technology overcomes the challenge of obtaining sufficient quantities of purified antigenic components due to the fact that multiple host organisms are able to produce the same antigen simultaneously.
However, a significant challenge in developing new immunization strategies involves designing vaccines that elicit the appropriate immune response to confer immunity against intracellular pathogens, particularly those that establish chronic, often lifelong infections. To achieve this, a thorough understanding of the antigens involved in pathogenesis and the immune protection mechanisms is required to rationally design vaccine strategies capable of surpassing the low protective immunity typically generated by infection [4].
How do AI and recombinant technologies work together for vaccine development?
Traditionally, antibodies have been generated using a single antigen species, as was done in the case of designing the initial COVID vaccine based on an animal model targeting the current strain. However, when a virus mutates, this method falls short, as it requires repeated injections to keep up with the rate of mutation.
To address this issue, computational technology can be used to collaboratively experiment and virtually compute strains from the beginning of a pandemic to the present day, resulting in over 1000 different hypothetical strains. Within hours, this technology can generate antibodies that target these various strains. Additionally, the same algorithm can be used to identify commonalities among mutant strains to extrapolate an antibody that can even target future strains. By studying the evolutionary traits of viral species, patterns can be identified to help predict future mutation trends. An algorithm can be created to analyze a virus’s evolution and identify common immunogenetic sequences that can guide vaccine design. This approach could significantly accelerate the vaccine design process and even uncover new biological phenomena in the future.
What role has Sino Biological played in promoting vaccine development?
Sino Biological has over 15 years of expertise in recombinant protein expression, and has established a proprietary mammalian transient expression platform that employs HEK293 and CHO cell lines adapted to serum-free medium or adherent cultures as the primary production system. This platform facilitates high-throughput delivery and the production of high-quality recombinant proteins and antibodies that are commonly used in antigen preparation, antibody discovery, protein structure determination and protein function research. Since proteins are an essential component of pathogens, and the targets that the immune system recognizes and attacks, Sino Biological is constantly researching, producing and optimizing potential vaccine targets of the future.
What exciting developments can we expect to see in this field in the future?
While existing platforms for vaccine development have limitations, ongoing efforts are focused on improving or building upon them. One area of significant potential is the development of new adjuvants, which until recently have mostly resembled those used in vaccines since the 1930s. Adjuvants play a crucial role in boosting immune responses and improving vaccine efficacy, making them particularly important for vulnerable populations, such as the elderly, who may have reduced immune function. One COVID-19 vaccine candidate, Novavax’s NVX-CoV2373, which is currently undergoing Phase III testing, combines a full-length SARS-CoV-2 spike protein in a recombinant nanoparticle with a saponin adjuvant and has demonstrated high efficacy [5]. Derived from the bark of a Quillaja saponaria tree, saponin adjuvants have been shown to invigorate both the humoral (antibody-related) and cell-mediated branches of the immune system in its response to a vaccine antigen. In particular, adding saponin to a vaccine leads to an unleashing of pro-inflammatory cytokines, like interleukin-1 and interleukin-6, which in turn further promotes antibody and memory cell production and further immune cell activation. Overall, the strengthened immune responses instigated by adjuvants can help to enhance the effectiveness of vaccines and improve protection against infectious diseases.